503A vs. 503B Pharmacies: What’s the Real Difference — and Why It Matters For Personalized GLP-1 & GIP Weight Loss Treatments
- katie7291
- Oct 8, 2025
- 4 min read
Updated: Oct 12, 2025
If you’ve been exploring GLP-1 and GIP-based weight-management therapy, you’ve likely seen terms like 503A and 503B pharmacies mentioned by clinics or compounding providers. These refer to two distinct types of FDA-regulated compounding entities defined under the Federal Food, Drug, and Cosmetic Act.
Both play valuable roles in healthcare, but they operate very differently — and that difference directly affects how your compounded GLP-1/GIP medication is prepared, dispensed, and personalized to your treatment plan.
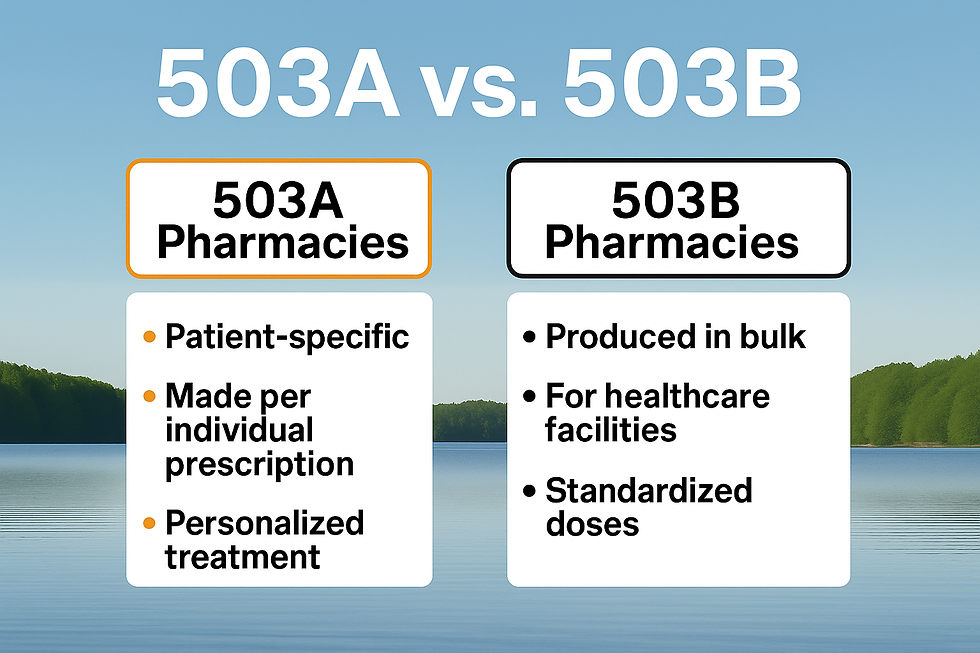
How Compounding Models Affect Personalized GLP-1 & GIP Treatments
A brief understanding of compounding models helps explain how personalized GLP-1 and GIP therapies are safely prepared for each patient. These models — 503A and 503B — define who compounds the medication, when it’s made, and under what level of oversight
503A Compounding Pharmacies and Their Role in Personalized GLP-1 and GIP Prescriptions
A 503A compounding pharmacy prepares medications only when a licensed prescriber writes a prescription for an individual patient. Each formulation is made in small quantities or as a single unit, under the supervision of a licensed pharmacist.
These pharmacies are primarily regulated by state boards of pharmacy, follow U.S. Pharmacopeia (USP) compounding standards, and must adhere to applicable FDA guidance.
503A compounding allows prescribers to tailor a therapy to a patient’s specific needs — which is especially relevant in weight-management protocols involving GLP-1 / GIP agonists, where dosing schedules and tolerability can vary between patients.
Examples of what a 503A pharmacy may compound include:
Adjusting GLP-1 / GIP dosage strength under provider supervision
Preparing formulations without certain excipients or dyes for patients with sensitivities
Creating alternative dosage forms (for example, oral vs. sublingual) when appropriate and prescribed
Combining additional supportive ingredients as directed by the prescriber
Every compounded prescription is made specifically for one patient and one prescription — never in mass batches.
What a 503B Outsourcing Facility Does
A 503B outsourcing facility functions more like a pharmaceutical manufacturer. It’s registered with the FDA and can compound medications in bulk, without requiring individual patient prescriptions. These facilities primarily serve hospitals, surgical centers, and large healthcare systems that need standardized sterile medications available at scale.
503B facilities must comply with current Good Manufacturing Practice (cGMP) — the same strict production and quality control standards that apply to large drug manufacturers. Their focus is consistency and supply, not personalization.
In the context of GLP-1 / GIP therapies, 503B facilities are typically the source of bulk-compounded formulations distributed to clinics that administer them under their medical protocols. These medications are not customized for an individual patient but are still made under strict FDA manufacturing oversight.
Why Telehealth Providers Often Partner with 503A Pharmacies for GLP-1 and GIP Weight-Management Programs
While both 503A and 503B operations maintain quality and safety controls, their intent and process differ.
503A pharmacies work directly from a prescription written for one patient, allowing pharmacist-to-provider communication and individualized compounding.
503B facilities produce larger quantities of standardized medications that can be dispensed to healthcare providers for office administration or resale.
Both models are legal and valuable. The choice between them depends on whether the therapy is meant for personalized use or institutional supply.
Why Personalized Weight-Loss Therapy Often Uses 503A Compounding
GLP-1 / GIP medications can be complex — dosing, titration schedules, and patient tolerance vary widely. That’s where 503A pharmacies play a critical role.
Under a provider’s direction, a 503A compounding pharmacy can:
Provide precise dosing adjustments prescribed for a patient’s specific stage of therapy.
Offer flexibility in formulation based on clinical need and tolerability.
Maintain direct oversight by the prescribing clinician and compounding pharmacist for every fill.
This individualized approach allows for prescription-by-prescription customization while staying within the boundaries of FDA and state pharmacy regulations.
When 503B Facilities Are Appropriate
For institutional or high-volume clinical settings — for example, clinics that administer standardized injectables on-site — 503B outsourcing facilities are indispensable.
They ensure consistent supply, uniform concentrations, and large-batch availability for procedures or injection programs. 503B compounding supports access and stability but does not provide individualized formulation per patient.
Putting It All Together
503A pharmacies focus on personalized compounding — one patient, one prescription, one pharmacist’s oversight.
503B facilities focus on scalable manufacturing — large batches intended for distribution to clinics and hospitals.
Both are regulated, both are necessary, and neither is inherently “better.” The distinction lies in whether your therapy calls for individualized customization or ready-to-use standardization.
The Bottom Line
When your provider prescribes a compounded GLP-1 / GIP medication through a 503A pharmacy, it means the formulation is being created specifically for you — under your prescriber’s instructions and a pharmacist’s supervision.
When a clinic sources its medications from a 503B outsourcing facility, it ensures consistent, large-scale availability for patients receiving standardized dosing programs.
Understanding the difference empowers you to make informed decisions about your care, know how your medication is being prepared, and appreciate the safeguards behind every prescription.
Disclaimer: Compounded GLP-1 and GIP medications are prescribed only when a licensed healthcare provider determines an individualized need. Compounded formulations are not FDA-approved but are prepared in accordance with applicable federal and state laws governing pharmacy compounding. This content is for educational purposes only and does not constitute medical or legal advice.



Comments